Whether you’re formulating/manufacturing drug substance or drug product, conducting clinical trials, or running internal audits of user/patient information, clearly documented and easy-to-understand procedures are vital to guide your team through complex processes. That’s where a Quality Management Plan (QMP) comes in.
A concrete QMP will not only provide a roadmap to comply with federal regulations, reduce errors and inefficiencies, and maintain consistent quality, it will also set expectations and keep everyone on the same page as you scale up.
What is a Quality Management Plan?
A Quality Management Plan is a guiding document that outlines the processes and standards of a project or program to establish a model of excellence. The plan defines what constitutes quality at every stage of the project and establishes a formal process for verifying quality at various stages.
It also identifies who is responsible for each stage as well as how to document the progress for compliance. It’s one thing to have a repeatable, high-quality go-to-market strategy for your new medical device. It’s another to be able to quickly and easily prove to auditors that everyone on your team followed the process exactly. By creating and following a QMP, all documentation and sign-offs at each step will be at your fingertips.
QMPs are critical for industries with significant regulation and high standards for performance. When bringing a medical device to market, for example, you’ll want to know early on if diagnostics don’t meet standards for precision, so they can be adjusted before affecting user testing and well before submission for regulatory approval.
When creating a QMP for a new project, you need to gather and create documentation that defines the standards for quality. Your QMP will also define roles, responsibilities, training, and timelines, while also embedding check-ins to ensure the project is meeting those standards.
So, to help scale effectively, what should your QMS include?
Document Your Quality Expectations for the Project
Before you even start building out quality management plans for every project, you’ll first need to clarify what capital-Q quality means for your company. Each QMP you create needs to include relevant documentation your team can refer back to at any time, as well as how to proceed with questions or concerns that aren’t covered. Store these documents in a place all stakeholders have access to, like an electronic quality management system (eQMS).
Overarching Company Quality Policy
Every QMP your company creates should include the organization’s quality policy, which itself should outline the company’s commitment to excellence and continuous improvement. This is the starting point for any standards around your project. All other documentation will vary depending on the scope of your project.
Applicable Regulations
Your QMP should include any federal, environmental, or industry regulations that apply to your project. Collecting this information upfront will help verify that you meet each one as you progress through your project, which in turn will make approvals go faster.
Some overarching regulations will apply to every project your company launches, but there may be variations depending on project type. Be sure to review applicable regulations before each project to make sure you have the necessary ones included in your QMP.
Market Research Documentation
Make it easy to cross-check market research to verify that the project aligns with consumer demand. Include any feedback or market analysis data that inspired the project so you can quickly check for scope creep. For example, if your users have asked for a simple interface for an existing medical device, adding additional features might not be valuable to your market.
Quality Criteria Definitions & Prioritization
As part of your QMP, define what success means for this project and document your priorities. For example, perhaps 100% regulatory compliance is more important than staying on budget, but staying on budget is more important than staying on schedule.
In this case, your QMP should clearly define the acceptable margins of your success metrics. How much over or under your target metrics, such as your budget, is permissible before changes need to be made? Is being over budget by 5% okay, or do you need to get approval for those additional costs? Having these numbers defined at the onset will keep you from having to stop and adjust targets throughout the project.
eQMS Tip: Use your Quality Management System to house all the documentation for your QMP, so all the information is easily accessible by all stakeholders.
An eQMS can simplify the documentation required for an effective QMP. Source: ZenQMS
Outline responsibilities and assign roles
Once you’ve documented what quality means for the project, identify and document who is responsible for executing the different stages of the project. By defining clear roles, you also create a communication chain of command, so everyone involved knows who to go to with questions. This avoids unnecessary slowdowns while team members cycle through stakeholders to get the information they need.
Identify the stakeholders for the project. This includes the people who will be doing the work, as well as the knowledge experts who need to be consulted, and the leadership team members who need to be kept informed.
Identify who will be accountable for each stage of the project. It’s important for the entire team to be able to see how their role fits into the larger scope of the project. Having these roles outlined in the QMP also allows people to see who they need to contact if there’s a question about a particular part of the project.
Identify deadlines for each stage. As you establish the timeline for the project, work through a schedule your team can achieve. Then build in a little margin at stages where issues might arise, such as material procurement or prototype approval.
eQMS Tip: Use your QMS to assign granular permissions and create subtasks for easier management. Automate follow-up tasks to keep the project moving smoothly.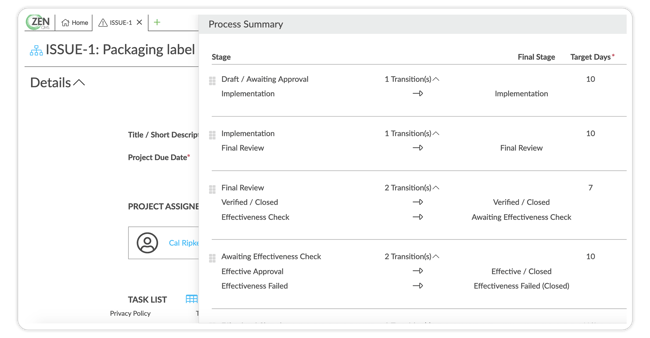
An eQMS makes it easy to manage QMPs by assigning permissions and follow-up tasks. Source: ZenQMS
Include Quality Checks at Every Stage
The goal of a Quality Management Plan is to eliminate errors. But small mistakes can often fall through the cracks as business scales up and more moving parts are added to each project. Having quality checks embedded in the QMP will provide peace of mind, as problems can be addressed before they derail the project.
Refer to the list of regulatory, environmental, and other requirements included in your quality documentation and create a checklist for each stage of the project. Include a step in your QMP that verifies that each regulation has been met before that project stage is complete. For example, check off any environmental regulations at the procurement stage when sourcing and storing batteries for your medical device and again during development to make sure your processes align with those same environmental requirements. Acknowledging and verifying the regulations at each stage will help you catch any issues early in the process. Documenting this along the way will make it easy to gather information for external auditors.
Your QMP should include opportunities to compare benchmarks with the quality criteria you defined earlier in the process. Set them against the metrics identified at the beginning to make sure that, as your team and project scope grows, everything is still on target.
eQMS Tip: Use your QMS to manage the lifecycle of your project documentation so everyone can clearly see progress and iterations. With built-in digital signatures and global search functions, all stakeholders can track what changes have been made and by whom.
Quality Processes Build Trust
In complex, heavily-regulated industries, quality must be at the heart of your business because your reputation depends on it. By creating quality management plans, you make sure your processes are followed and quality is the standard. This extra layer of quality control cuts down on errors and becomes a competitive advantage.
If you are ready for an eQMS that makes it easy to build quality into your processes, reach out for a demo with ZenQMS today.

